|
.
AG Gründker
|
|
|
Metastasierung
|
.
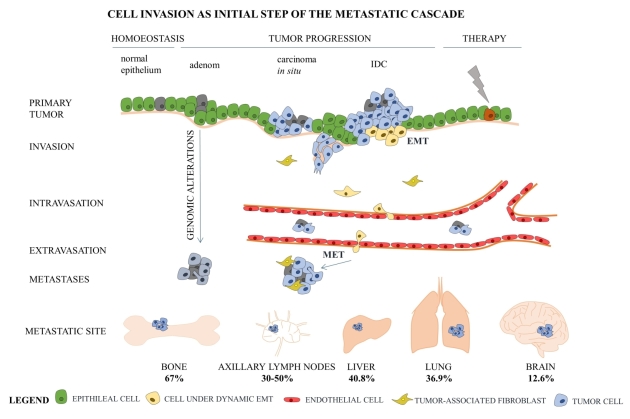
Das erste Schlüsselereignis im mehrstufigen Prozess der Metastasierung
ist die Ablösung der Tumorzellen vom Primärtumor und die Ausbreitung in
das umgebende Gewebe. Die Tumorzellen erlangen die Fähigkeit zu wandern
und in Nachbargewebe einzudringen, indem sie ihre Zytoskelett-Organisation, Zell-Zell-Kontakte, Kontakte mit der extrazellulären Matrix (ECM) und dem umgebenden Stroma verändern. Die epithelial-mesenchymale Transition
(EMT) ist ein transientes dynamisches Programm, das durch verschiedene
Transkriptionsfaktoren (TFs) induziert wird. EMT-TFs regulieren
tumorfördernde Veränderungen der Mikroumgebung,
Krebsstammzelleigenschaften und Therapieresistenz. Der Beitrag von
EMT-Programmen zur Metastasierungskaskade bei Brustkrebs wird durch
viele Publiaktionen unterstützt. Es wird jedoch noch diskutiert, ob
eine Beteiligung von EMT-Programmen für die Schaffung eines invasiven
Phänotyps unabdingbar ist. Daher ist es notwendig, die Invasion von
Krebszellen im Hinblick auf die EMT-Komplexität weiter zu untersuchen.
Publikationen:
- Kolb
K, Hellinger J, Kansy M, Wegwitz F, Bauerschmitz G, Emons G,
Gründker C (2020) Influence of ARHGAP29 on the invasion of
mesenchymal-transformed breast cancer cells. Cells 9(12): 2616
-
Hellinger
JW, Schömel F, Buse JV, Lenz C, Bauerschmitz G, Emons G, Gründker C
(2020) Identification of drivers of breast cancer invasion by secretome
analysis: insight into CTGF signaling. Scientific Reports 10(1):17889
-
Hellinger JW, Hüchel S, Goetz L, Bauerschmitz G, Emons G, Gründker
C (2019) Inhibition of CYR61-S100A4 axis limits breast cancer
invasion. Frontiers in Oncology 9:1074
-
Gründker C,
Läsche M, Hellinger JW, Emons G (2019) Mechanisms of metastasis
and cell mobility: The role of metabolism. Geburtshilfe und Frauenheilkunde 79: 184-188
-
Gründker C, Emons G (2017) The Role of Gonadotropin-Releasing Hormone in Cancer Cell Proliferation and Metastasis. Frontiers in Endocrinology 8:187
-
Gründker
C, Bauerschmitz G, Schubert A, Emons G (2016) Invasion and increased
expression of S100A4 and CYR61 in mesenchymal transformed breast cancer
cells is downregulated by GnRH. International Journal of Oncology 48(6):2713-2721
-
Gründker C, Bauerschmitz G,
Knapp J, Schmidt E, Olbrich T, Emons G (2015) Inhibition of SDF-1/CXCR4
system-induced epithelial-mesenchymal transition by kisspeptin-10. Breast Cancer Reseach and Treatment 152(1):41-50
-
Ziegler E, Hansen M-T, Haase M, Emons G, Gründker C (2014) Generation of MCF-7 cells with aggressive metastatic potential in vitro and in vivo. Breast Cancer Research and Treatment 148(2):269-277
-
Schmidt E, Haase M, Ziegler E,
Emons G, Gründker C (2014) Kisspeptin-10 inhibits stromal-derived factor-1-induced
invasion of human endometrial cancer cells. International Journal of Gynecological Cancer 24(2):210-217
-
Ziegler E, Olbricht T, Emons G, Gründker
C (2013) Antiproliferative effects of kisspeptin-10 depend on
artificial GPR54 (KiSS1R) expression levels. Oncology Reports 29(2):549-554
-
Schubert A, Hawighorst
T, Emons G, Gründker C (2011) Agonist and antagonists of GnRH-I and -II reduce metastasis
of triple-negative human breast cancer cells in vivo. Breast Cancer Research
and Treatment 130(3):783-790
-
Olbrich T, Ziegler E, Türk G, Schubert A, Emons
G, Gründker C (2010) Kisspeptin-10 inhibits bone-directed migration of GPR54-positive
breast cancer cells: evidence for a dose-window effect. Gynecologic Oncology 119:571-578
-
Schubert A, Schulz
H, Emons G, Gründker C (2008) Expression of OPG and RANKL in HCC70 breast cancer cells
and effects of GnRH treatment on RANKL expression. Gynecological
Endocrinology 24(6):331-338
-
von Alten J, Fister
S, Schulz H, Viereck V, Frosch KH, Emons G, Gründker C (2006) GnRH analogs reduce
invasiveness of human breast cancer cells. Breast Cancer Research and Treatment
100:13-21
|
|
|
|
|

